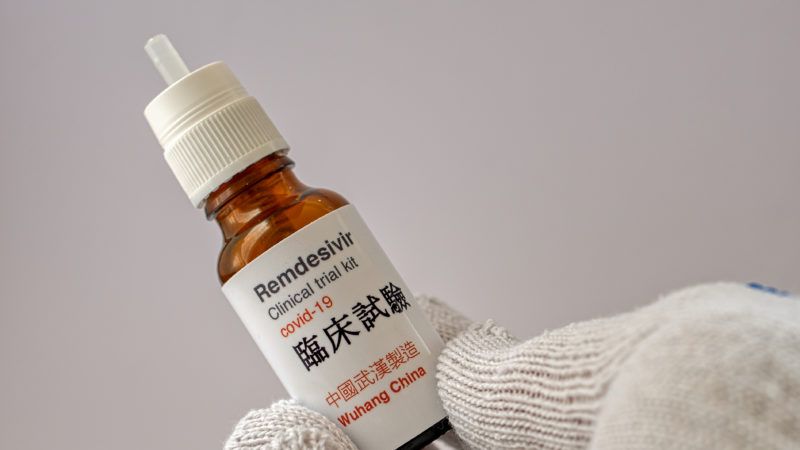
Gilead Science Donates 1.5m Vials Of COVID-19 Drug To U.S
Gilead Science Inc, maker of the coronavirus drug Remdesivir said it will be donating 1.5 million vials of the drug to help patients in U.S.hospitals.
Chief Executive of the company Daniel O’Day made the pledge during a meeting with President Donald Trump on Friday.
The meeting came after the drug was granted emergency use authorization by the U.S. Food and Drug Administration for COVID-19.
The FDA approval cleared the way for broader use of the drug in more hospitals around the United States.
But the authorization applies to patients hospitalized …